Elements and Compounds
Elements and Compounds: Overview
This topic covers concepts, such as, Pure Substances, Metals,Properties of Non-Metals and Non-Metals etc.
Important Questions on Elements and Compounds
Antimony is a metal.
Name the metalloids in the first twenty elements.
Chemically matter is classified into pure substance and _____.
Write a note on the chemical classification of matter.
Metalloids are insulators.
List one property of metalloid.
_____ is an example of metalloid.
Metalloids have the properties similar to that of only metals.
Consider the elements of group-14. Choose the correct alternative:
a) Si and Ge are semiconductors
b) Carbon and silicon are non-metals
c) Sn and Pb are metals
d) Si and Ge are metalloids
Which of the following statements are false for pure substances?
(i) pure substances contain only one kind of particles.
(ii) Pure substances may be compounds or mixtures.
(iii) Pure substances have same composition throughout.
(iv) Pure substances can be exemplified by all element other than copper.
Pure substances have fixed melting and boiling points.
What are metalloids? Explain the uses of some metalloids.
Give one or two word(s) for the following:
A pure substance made up of only one kind of particles.
What is a compound?
Identify the state of matter based on the arrangement of the molecules.
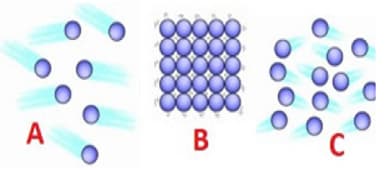
Matter is composed of
Pure substances are homogeneous substances, in which only one type of molecule is present.
Pure substances are those substances
Name the property of graphite that makes it an exceptional non-metal.
A pure substance consists of only one type of
